After administration of autologous virus-specific T cell therapy (LB-DTK-COV19), it was confirmed that there were no adverse reactions or side effects and that the COVID-19 pneumonia was completely cured.
It is news that a seriously ill patient suffering from persistent pneumonia after the onset of COVID-19, with coronavirus detected for a long time despite the use of antiviral treatments, was successfully treated with the administration of viral T-cell therapy antigen-specific . This is the first case of treating a patient with long-term COVID-19 infection, for which there was no cure, with a domestically developed cell therapy.
Immunocompromised patients with COVID-19, including patients with blood cancers such as malignant lymphomas, autoimmune diseases taking immunosuppressants, and patients with HIV infection, have limited humoral immunity due to antibody production, which also prevents viral replication when antiviral treatment is given. Expulsion cannot be prevented. If this infection persists for a long time, it can progress to severe pneumonia and ultimately lead to death.
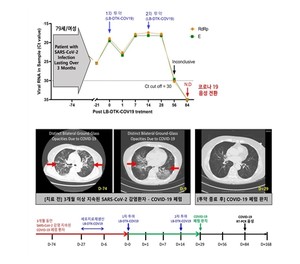
Professor Lee Rae-seok’s research team from the Department of Infectious Diseases at Seoul St. Mary’s Hospital of the Catholic University of Korea announced that it has administered autologous virus-specific T cell therapy to two patients suffering from HIV infection. “SARS” for a long time. CoronaVirus-2′. One of them was a 79-year-old patient being treated for blood cancer (lymphoma). After being infected with COVID-19, he still did not respond to prescriptions for antiviral drugs. The virus was detected for 3 months, progressed to severe pneumonia and I finally stopped chemotherapy. I’m a patient.
The research team used a coronavirus-specific multi-antigen T-cell therapy produced by LucasBio (CEO, Professor Cho Seok-gu), a cell therapy company from the same university. After collecting the patient’s blood, we challenged it with three multiple antigens (Spike, Nucleocapsid, and Membrane) known to be the major antigens of the SARS coronavirus to produce a coronavirus antigen-specific T cell therapy capable of responding to mutations and administered twice and was administered. Subsequently, for the objective evaluation of clinical recovery, the WHO Corona Patient Rating Scale (ordinal scale), Symptom Recovery Measurement Score (NEWS2 score) and chest CT were used to verify the degree of recovery from pneumonia.
As a result, all long-infected patients tested negative in the PCR test, and the clinical symptoms such as cough, fever and breathing difficulties caused by the coronavirus disappeared significantly, and all oxygen treatment was stopped. Furthermore, on the final chest CT, the shaded ground-glass nodules (which appear cloudy on the CT as if they were ground glass) disappeared, confirming that the severe pneumonia was completely healed.
Korea declared a “COVID-19 pandemic” in August last year, but coronavirus infections, including the outbreak of a new coronavirus, are still ongoing. Even if healthy adults are infected, the immune response of T cells in the immune system suppresses the proliferation of the virus and helps eliminate it, but in immunocompromised people the immune system does not function.
Furthermore, it is known that antiviral drugs can reduce mortality rates and severity, but in patients with a weakened immune system due to chemotherapy or for various reasons, the replication and excretion of the virus continues even if antiviral treatment is administered . This causes an inflammatory reaction that can progress to pneumonia, severe respiratory failure and pulmonary fibrosis, leading to death. Indirect harmful reactions are also increasing, such as the delay in life-related treatment of seriously ill patients, such as anticancer treatment, or the possibility of the emergence of coronavirus variants due to long-term infections and the increased possibility of transmission to others . .
Professor Lee Rae-seok said: “This product was produced by challenging with multiple SARS-Coronavirus-2 antigens using autologous immune cells targeting severely immunocompromised patients who had no treatment options, patients refractory to existing treatments, and COVID patients -19 long term. “This is Korea’s first treatment using cell therapy,” he said of the research findings.
Professor Lee continued: “As a result of analyzing the patient’s blood to see how much the patient’s immunity, particularly the cellular immunity that responds specifically to the coronavirus, increased after the administration of virus-specific T-cell therapy , surprisingly, gradually increased after 7 days.” “We have confirmed that it is directly related to clinical recovery indicators, and through further analysis, we will discover the immunological mechanism and expand it to treat not only coronavirus but also severe respiratory viral infections,” he said.
This study was selected for the first Advanced Regenerative Medicine Clinical Research Support Project hosted last year by the Government Advanced Regenerative Medicine Clinical Research Support Project, titled “LB-DTK-COV19 self-administered to long-term infected patients with SARS-Coronavirus-2.” This is a single-center phase 1 and 2 clinical trial to evaluate the safety and efficacy of. With the participation of Professor Lee Dong-gun (co-investigator) from the Department of Infectious Diseases of Seoul St. Mary’s Hospital, Professor Seok-gu Cho (co-investigator) from the Department of Hematology and Dr. Na-yeon Kim and Dr. Geon-il Lim (co-investigators, cell therapy development) from Lucas Bio, we will continue to treat patients with long-term COVID-19 infection for further clinical research.
#Autologous #Tcell #antiviral #therapy #treats #severe #selfinfected #COVID19 #patients