A research team at Argonne National Laboratory is introducing a redox-active intermediate layer into a lithium-sulfur (Li-S) battery, dramatically increasing energy density and cycle life, which have been challenges for successful commercialization.
The results of the research were published in the journal Nature Communications on August 8, 2022.
Currently, lithium-ion batteries are used in various places such as mobile phones and electric vehicles. However, lithium-ion batteries also have disadvantages such as short life, easy overheating, and difficult raw material supply. As an alternative battery, the Li-S battery, which has a much higher theoretical energy density, is rich in sulfur as a raw material, and is cheap, expected and developed.
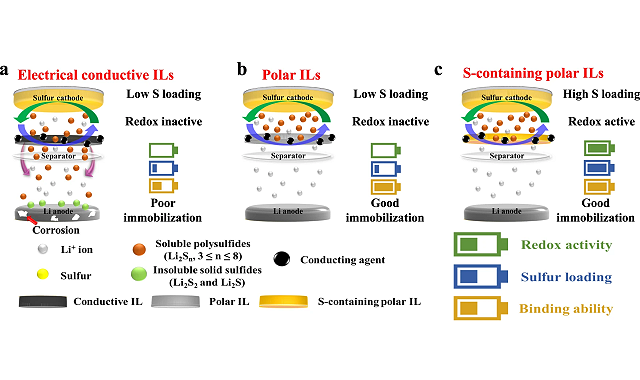
A Li-S battery has a structure where an organic electrolyte is sandwiched between a positive electrode containing sulfur and a negative electrode made of lithium metal. However, due to the shuttle effect in which the polysulfide, the reaction intermediate of the positive electrode, dissolves in the electrolyte and moves to the negative electrode, self-discharge progresses and the charging rate decreases , and the battery life is shortened.
In order to prevent the polysulfide shuttle effect, previous studies have attempted to place a redox-inert protective interlayer between the positive and negative electrodes. However, the protective interlayer makes the battery even heavier, reduces the energy storage capacity per unit weight of the battery, and cannot sufficiently prevent the shuttle effect, which has been a major obstacle to the application of batteries Li-S is practical.
The research team developed an interlayer containing porous sulfur with redox activity and introduced it to Li-S batteries. The battery exhibited an initial capacity approximately three times higher than that with an inert interlayer, and retained 64% of its initial capacity over 700 charge cycles. They found that the active oxidation-reducing interlayer protects the lithium negative electrode, electrochemically regenerates the polysulfide dissolved in the electrolyte, and increases the battery’s capacity.
In the future, the research team hopes to improve the performance of Li-S batteries by further developing interlayer technology with redox activity, making the interlayer thinner and lighter, and promoting practical application.
Relevant information
Development of high-energy non-aqueous lithium-sulfur batteries through a redox-active interlayer strategy | Nature Communication
Lithium-sulphur batteries are one step closer to powering the future | Argonne National Laboratory