10 times reduction in precious metal catalyst consumption through electrode interface control
[인더스트리뉴스 이건오 기자] While various technologies for the production of hydrogen are proposed, ‘green hydrogen’ technology, which does not emit carbon dioxide at all, is attracting attention.
In particular, water electrolysis technology, which produces hydrogen and oxygen by electrolyzing water, has excellent stability in renewable energy power systems with high volatility, drawing attention as a next-generation system that will be responsible for the growing demand for green hydrogen. in the future.
KAIST (President Lee Kwang-hyung) has identified a performance degradation phenomenon that occurs when the anode noble metal catalyst content is reduced in a polymer electrolyte water electrolysis system that uses a thin polymer membrane as a separator by Professor Hee’s research team -Tak Kim in the Department of Biochemical Engineering It was announced on May 22 that she had found .
The results of this research, in which Dr Ki-soo Doo from the Department of Chemical and Biomolecular Engineering at KAIST participated as the first author, were published as the cover paper of the online edition of the international journal ‘ACS Energy Letters’ on May 12.
Water electrolysis cation conductive polymer electrolyte is an eco-friendly hydrogen production device that electrolyzes water to produce hydrogen gas.
This water electrolysis system operates in an acidic environment and uses a noble metal catalyst for efficient water splitting. However, precious metal materials such as platinum and iridium pose problems of shortage of supply and high price. In particular, an iridium-based catalyst is most suitable for the anode reaction, but there is a need to develop a polymer electrolyte water electrolysis device that requires one-tenth of the current catalyst due to its low reserves.
However, the rapid performance degradation that occurs when the iridium catalyst content is reduced holds back the reduction in the price of polymer water electrolyte electrolysis. Most of the research to solve these problems is focused on finding new catalysts that can replace iridium.

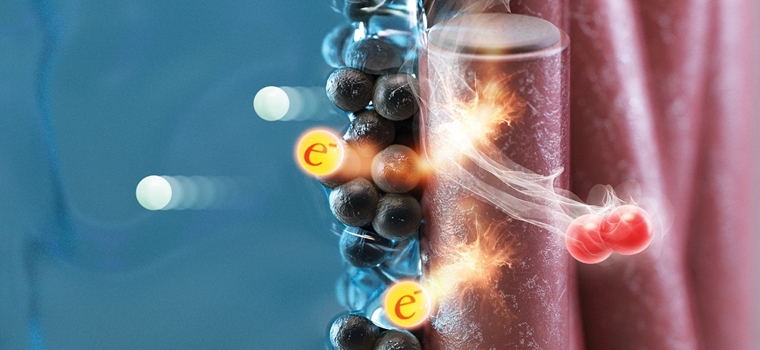
The electrode used in the water electrolysis system has a structure where a catalyst layer consisting of an iridium catalyst and a binder and a titanium diffusion layer are combined. Professor Hee-Tak Kim’s research team presented a new perspective and pointed out that the performance degradation problem that occurs when the content of iridium catalyst in the anode of PEM water electrolysis is reduced due to the increase in the content of the binder at the interface between the catalyst layer and the diffusion layer.
When the iridium catalyst and the titanium diffusion layer come into contact, a band bending phenomenon occurs where the electron band of the natural oxide film present on the titanium surface is bent. According to the results of the research team, in electrodes with a low iridium content, this band bending phenomenon is amplified by the binder. The more the electron strip is bent, the more difficult it is to transfer electrons, leading to performance degradation.

The research team confirmed that the same water electrolysis performance can be obtained even when the iridium content is reduced to 1/10 level when designing an interface with reduced band bending. This proves that the amount of expensive noble metal catalyst used can be significantly reduced by changing the composition of the electrode interface.
Professor Kim Hee-tak said, “The results of this study are significant as they pointed to the problem of the performance of the water electrolysis electrode with less iridium, which was covered with a coating, identified the cause, and provided an answer strategy. ” Based on this, efficiency and price We hope it will be applied to the development of a green hydrogen production system that can hold both at the same time.”
Copyright © Industry News Unauthorized reproduction and redistribution prohibited







.jpg?fit=300%2C300&ssl=1)

